Non-thermal microwave effects during microwave
processing of clay minerals
G. Link1,
W. Faubel2, St. Heissler2, P.G. Weidler2, M.
Thumm2,3
Forschungszentrum
Karlsruhe, 1IHM, 2ITC-WGT, Karlsruhe Germany
3 and
Abstract
Investigations on processing of kaolinite clay minerals revealed that the phase transformation into meta-kaolinite happens much faster in case of microwave processing compared to conventional processing. To elucidate this behavior a special set-up for in-situ FTIR spectroscopy has been realized. This allows comparing IR transmission spectra of samples with and without the influence of the microwave field in situ. Preliminary results from these investigations indicate a selective microwave heating of hydroxyl groups of the kaolinite layered alumosilicate, what possibly explaines the microwave specific effect.
Keywords: microwave processing, FTIR spectroscopy, microwave effect, clay minerals, kaolinite
Intorduction
Microwaves
face growing industrial interest as a materials processing technology. It
allows instantaneous volumetric heating of dielectric materials leading to a
reduction of process time and therefore energy consumption in comparison to
conventional heating. This benefit is most distinctive for materials with low
thermal conductivity such as, polymers, glasses, powders or powder compacts.
Beside this procedural benefit very often a reduction of process temperatures
has been reported for various materials as well. Often such differences in
process parameters are used to argue for microwave-specific, non-thermal
effects, although very often this is a critical issue due to unknown or
underestimated temperature gradients within the material. Therefore novel
experimental methods would be preferable, which allow getting more detailed
information about the materials behaviour in microwave fields.
The potential of microwave technology for processing of various clay minerals has been investigated. One material under test was the aluminosilicate kaolinite. Kaolinite is a clay mineral widely used in ceramic, paper and chemical industries as an additive which gives specific properties to a large variety of products. To influence such characteristics and to develop new applications the kaolinite has to be modified in an appropriate way. One typical process for this material is the dehydroxylation reaction into the meta-kaolinite phase, which was investigated with microwave technology in comparison to the conventional process. One of the problems of the industrial process in fluidized bed reactors or rotary kilns is that usually the necessary heat is generated by combustion of fuel. But for the production of white pigments it is very important to make sure that the fuel burns without the generation of ash. The use of microwave technology is one potential solution to overcome this problem.
Experimenal
Kaolinite DSK50 from the Dorfner Company,
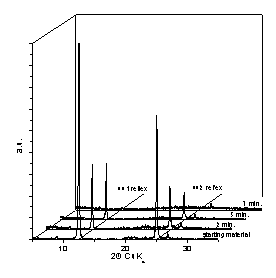
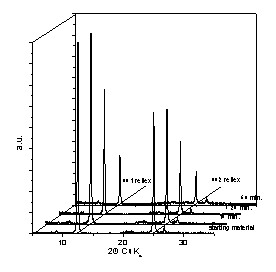
Fig. 1: XRD results for mm-wave processed (left) and conventionally processed (right) kaolinite at 600 °C after different processing time [1].
Increasing process temperature and/or processing time leads to a continuous disappearance of such diffraction peaks which happens much earlier in the case of mm-wave processing in comparison to the conventional process (see Fig. 1). During mm-wave calcination at 600 °C the diffraction peaks completely disappear after 7 minutes soak already, while with the conventional process even 60 minutes at 600 °C are not sufficient to complete the process. For verification that loss of crystallinity is due to a dehydroxylation reaction rather than due to a delamination process the specific surface of the powder material has been characterized by the BET method. The specific surface area of the processed powders was found to be comparable to the one of the starting powder, clearly indicating that delamination could be ruled out, hence the dehydroxylation reaction into an amorphous meta-kaolinite had occurred.
In order to get more confidence that the
observed reduction in processing time is a real microwave specific effect and
not based on potential errors in temperature measurement or based on
underestimated temperature gradients, further investigation in combination with
a FTIR spectrometer where realized. Therefore a simple TE103
waveguide resonator has been assembled that could be integrated into a BRUKER
IFS66 FTIR spectrometer. This experimental setup as shown in Figure 2 allows
gathering IR transmission spectra in the range from 400 cm-1 to 4000
cm-1 under the influence of electromagnetic fields at 2.45 GHz. In
order to reduce the absorption bands coming from CO2 and water
vapour in the atmosphere the whole course of the IR beam was covered and
continuously purged with dry air. For measurement of transmission spectra the
powder materials under test were mixed in low concentrations of about 1 to 2
weight% with KBr powder. In order to produce a transparent sample for
transmission measurements, out of these powder mixtures pellets were made under
a pressure of 10 tons in a laboratory press. Under this high load the KBr takes
a glass like state with the sample distributed homogenously inside. The
dimensions of so preserved pellets are 13mm in diameter and about 1mm in
thickness. A background spectrum was obtained by measuring a pure KBr pellet.
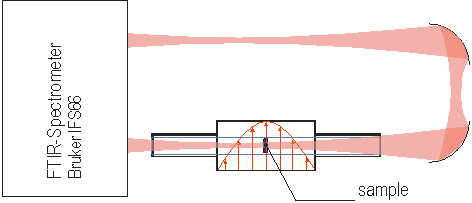
Fig. 2: Bruker IFS66 FTIR spectrometer in
combination with a single mode waveguide resonator.
The
sample temperature in this setup was limited by the thermal stability of the
PTFE sample holder and the fibre optic temperature
sensor (OPTOcon GmbH,
First
experiments were performed at ambient bulk temperatures in the following way.
IR transmission spectra were recorded with microwave power switched on for 1 or
2 seconds, sufficiently long to measure 3 to 4 interferograms used to calculate
the IR spectrum and sufficiently short to avoid significant sample heating.
Thereafter another spectrum was recorded with microwave power switched off.
This was repeated 10 times. Then the average spectra for both conditions
(microwave on and off) were compared in accordance to the experimental
approach, published by M. Vala and J. Szczepanski [2]. Figure 3 gives the
average spectrum when the kaolinite sample was exposed to microwave radiation
(red line) as well as the difference to the spectrum without microwave
irradiation (blue line).
The noisy
spectral ranges in the difference spectrum with wave numbers above 3500 cm-1
and from 1300 cm-1 to 2000 cm-1 are mainly dominated by
free water. Furthermore some absorption bands typical for kaolinite can be
distinguished in the difference spectrum as well. This indicates that heating
by microwaves is specific and must be different to conventional heating. All
absorption bands should be visible in the difference spectrum if the bulk
temperature of the sample changes, but this was not the case here. Assignment
of the bands in the difference spectrum yielded bands at 3697, 3620 and 914 cm-1.
For all three bands hydroxyl groups are involved. Beside the appearance of
three SiO vibraton bands at 1114, 1033 and 1006 cm-1, respectively,
many other bands from the kaolinite spectrum do not appear in the difference
spectrum. This indicates that microwave predominantly heats the hydroxyl groups
and therefore accelerates the dehydroxylation process into the amorphous
meta-kaolinite, as it was observed in Figure 1.
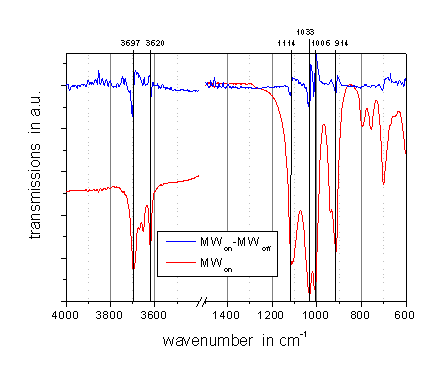
Fig. 3: IR spectrum of kaolinite at room temperature under microwave
irradiation (bottom), and enlarged difference spectrum to the non-irradiated
state (top).
Summary and outlook
The dehydroxylation process of kaolinite
into the amorphous meta-kaolinite was found to happen much faster in case of
microwave heating compared to conventional heating. In situ FTIR spectroscopy during
microwave heating was established as a new approach to get possible
explanations for such a microwave effect. First results from FTIR transmission
spectroscopy indicate a selective heating of the hydroxyl group in the
koalinite structure. In future this type of FTIR measurement is planed to be
extended to higher temperatures in a fully automated way in transmission as
well as in reflection. Another option will be the integration of the microwave
resonator into a Raman spectrometer.
References
- Link G., Hauser-Fuhlberg M., Janek M., Nüesch R., Takayama S., Thumm M., Weisenburger A.; High temperature processing of powders using millimeter-waves; Conf. Proc. of the 4th World Congress on Microwave and Radio Frequency Applications, Austin, Tex., November 7-12, 2004, New York, N.Y. : AIChE, 2005 S.54, ISBN 0-8169-0967-9.
- M. Vala, J. Szczepanski; A microwave effect: Molecular level microwave study of water vapour; in Ceramic Transactions Vol. 80, Microwaves: Theory and Application in Materials Processing IV; editors D.E. Clark, W.H. Sutton, D.A. Lewis, (1997), pp. 107-114.
- T. Wiechowski, A. Wiewiora, New approach to the problem of the interlayer bonding in kaolinite; Clays and Clay Minerals, Vol. 24, pp. 219-223. Pergamon Press 1976.