Recently,
the synthesis and application of functional magnetic nanocomposites, e.g. based
on silica-encapsulated nanomagnets have attracted increasing interest in catalysis
research. Such hybrid nanocomposite species reveal sustainable catalytic activities
and great advantages concerning catalyst recycling processes. For this purpose
magnetic particles with a large magnetization are required to achieve a facile
manipulation of the catalyst by an external magnetic field. Thus efforts have
been made to produce mesoscale spheres by encapsulating superparamagnetic
nanoparticles in a non-magnetic SiO2 matrix. Various sol-gel based
strategies have been used for generating silica-encapsulated iron oxide
particles.1 However, reports on silica-encapsulated magnetic metal
particles like Co, Fe, or Ni are scarce, even though many advantages are
expected (e.g., large saturation magnetization, enhanced magnetophoretic mobility).
We take
advantage of amino-functionalized siloxanes not only to directly control
particle nucleation and growth by coordinating to the metal surface but also to
provide reactive siloxane groups on the particle surface as a functional
interface for further deposition of oxides, such as SiO2 and TiO2.2
This procedure permits the synthesis of Co and Fe nanoparticles of various
sizes by thermolysis of Co2(CO)8 or Fe(CO)5 in
solution, respectively, and the preparation of mesoscale magnetic composite
particles. The reaction mechanism was investigated by UV-visible and FTIR spectrometry;
the size, structure, and magnetic properties of the particles were characterized
by TEM, EDX, XPS, Mössbauer spectroscopy, XRD, AES-ICP, and magnetic measurements.
Catalytic
magnetic microspheres were prepared by applying a three-step protocol: (1) The
APTES-functionalized Co nanoparticles (Fig.1a) were initially aggregated into
mesoscale spherical particles (Fig.1b). (2) A 30 nm-sized SiO2 layer
was then developed through surface APTES by using a sol-gel technique (Fig.1c).
(3) In the last step the catalytically active compound was deposited either by
developing an additional TiO2 layer (Fig.1d) or by immobilizing a
homogeneous rhodium catalyst (Fig.2).
a) b)



c)
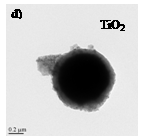
![]()
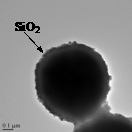
Fig. 1: TEM images showing Co@APTES-nanoparticles (a)
their spherical aggregates (b), Co@SiO2-microsphere (c) and TiO2
coated Co@SiO2-microsphere (d).
The activity, selectivity, and magnetic recycling
of the immobilized rhodium complex were investigated in hydroformylation
reactions using 1-octene as model substrate.
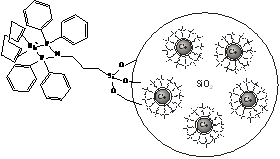
Fig. 2: Co@SiO2-immobilized
rhodium catalyst
TiO2
is an interesting photocatalyst used for the treatment of biological or organic
pollutants in water3. The photocatalytic activity of the TiO2
functionalized microspheres was investigated by using the decomposition of
methylene blue as a model reaction.


Fig. 3: Magnetic separation of the photocatalyst from
solution after degradation of methylene blue.
Acknowledgments
We acknowledge
Raphael Posselt, Sarah Essig, and Bernhard Powietzka for technical assistance.
References
[1] A.P.
Philipse, M. P. van Bruggen, C. Pathmammanoharan, Langmuir, 1994, 10, 92.
[2] A. Gorschinski, G. Khelashvili, D. Schild, W. Habicht, R. Brand, M. Ghafari, H. Bönnemann, E. Dinjus, S. Behrens, J. Mater. Chem., 2009, 19, 8829.
[3] X.
M. Song, J. M. Wu, M. Yan, Thin Solid Films, 2009, 517, 4341.